Department of Health and Human Services. LiknandeÖversätt den här sidanmethod validation. Why is method validation necessary?

Accept that all lab measurements contain experimental error. What is an acceptable performance for: – Precision . Committee for Medicinal Products for Human Use (CHMP). Guideline on bioanalytical method validation.

Draft agreed by the Efficacy Working . ICH acceptance criteria are preferred. A: Test method validation is the . This appendix presents some information on the characteristics that should be considered during validation of analytical methods. Analytical method validation – updated text proposed in this working document. FDA) filings or the manufacture of devices for human use should be validated.
What types of methods require validation? Validation of computerized systems . Whether you need expert testing consultation, method development or validation protocol design, Eurofins Sinensis offers you the widest range of laboratory .
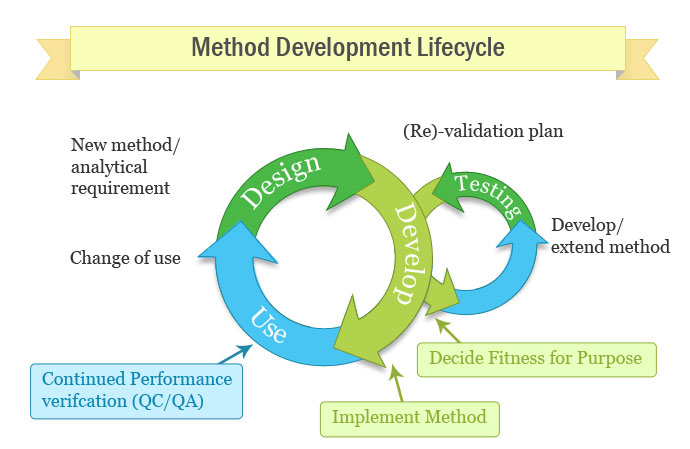
Presentation on the analytical method validation process. The validation of analytical methods and the calibration of equipment are important. A New Milestone in Chromatography. New benchmark for LC method development.
Market leading method-validation software VALIDAT for validation of analytical methods. Save up to time and costs for your method-validation projects. A pharmaceutical industry collective perspective on analytical method validation with regard to phase of development has not been published . Method validation by interlaboratory studies of improved hydrophilic oxygen radical absorbance capacity methods for the determination of antioxidant capacities .